Free Continuing Education for Oncology Nurses
The purpose of this 2-part module series is to provide an overview of oncology nursing, outlining the core aspects of cancer diagnosis, staging, and treatment and the role, responsibilities, and professional performance of oncology nurses. Part 1 will discuss the pathophysiology of cancer, early detection and prevention, and unique care considerations related to surgical and radiation oncology.
eness, and response to treatment. Cancer disparities are also exacerbated by a lack of diversity in clinical trial participation, as the results may not be applicable across populations. Furthermore, the COVID-19 pandemic limited access to cancer prevention, early detection, and treatment services. These delays in care are predicted to exacerbate existing cancer disparities due to the unequal burden the pandemic has exerted on all Communities of Color (ACS, 2022b, 2022c; NCI, 2020c).
Pathophysiology of Cancer
Cancer results from uncontrolled cell division and faulty mechanisms that allow cells to spread into surrounding tissues. Cancer cells have distinct features compared to healthy cells, such as their appearance under a microscope, growth and replication patterns, and overall functioning. When cancer cells collect in an area, they form a malignant (cancerous) tumor. Normal, healthy cells reproduce in an organized, controlled, and orderly manner as they mature into individual cells that serve specific functions and have predetermined lifespans. They undergo apoptosis (i.e., programmed cell death), where the body gets rid of unneeded cells. They do not divide when space or nutrients are limited and do not spread to other parts of the body where they do not belong. In contrast, cancer cells are less specialized, exhibit dysplasia (disorganized growth) and hyperplasia (increased size), and evade apoptosis as they divide and grow uncontrollably, even when space is limited. Cancer cells can travel through the bloodstream or lymphatic system to distant sites and develop new tumors. They can manipulate healthy cells to generate blood vessels (angiogenesis) that supply the tumor with oxygen and nutrients needed for growth. In addition, their duplicitous appearance helps them remain hidden, preventing the immune system from recognizing them as abnormal and eradicating them (Yarbro et al., 2018).
All cancer is inherently genetic, as cancer cells contain genetic mutations that lead to uncontrolled cell division and growth. Healthy genes comprise deoxyribonucleic acid (DNA) sequences that contain information necessary for proper functioning. Genetic changes linked to cancer growth typically affect three types of genes: proto-oncogenes, tumor-suppressor genes, and DNA repair genes. Proto-oncogenes make specific proteins involved in healthy cellular growth, division, and replication. When proto-oncogenes are altered (or mutated), they can become cancer-causing oncogenes, allowing cells to grow and replicate out of control. Likewise, tumor-suppressor genes also help regulate healthy cellular growth and division; thus, mutations in tumor-suppressor genes eradicate their ability to control cellular processes, leading to unrestricted cellular division. Finally, DNA repair genes are tasked with fixing damaged DNA to prevent cancer growth, as all cells in the human body are prone to DNA damage over time. However, if repair genes are damaged or mutated, the cell loses its ability to repair. Errors cultivate and replicate over time, leading to duplications and deletions of chromosomal components. Collectively, these mutations can provoke cancer (NCI, 2021b; Yarbro et al., 2018).
While all cancer is genetic, not all cancer is hereditary. Inherited (or hereditary) cancer occurs only when a damaged gene with high cancer susceptibility is passed through generations. In these cases, a patient with hereditary cancer is born with an abnormal genetic mutation and an increased predisposition to develop cancer at some point in their lives. Inherited mutations are also called germline mutations and appear in all cells in the body. Breast cancer genes 1 (BRCA1) and 2 (BRCA2) are among the most common inherited mutations linked to cancer. Everyone is born with BRCA1 and BRCA2 genes. In their physiologic form, these genes prevent cancer by regulating the growth and division of specific cell types. However, harmful changes in the BRCA1/BRCA2 genes lead to a loss of functioning. People who inherit mutated BRCA1/BRCA2 genes have an increased risk for several cancer types, most notably breast, ovarian, and prostate cancer. They also tend to develop cancer at younger ages (Centers for Disease Control and Prevention [CDC], 2021a; NCI, 2020a).
BRCA1/BRCA2 mutations account for up to 10% of cases of breast cancer. About 50 in 100 women with a BRCA1/BRCA2 mutation will be diagnosed with breast cancer by the time they turn 70 years old, compared to 7 in 100 women in the general population. Furthermore, nearly 30 in 100 women with a BRCA1/BRCA2 mutation will be diagnosed with ovarian cancer by their 70 th birthday, compared to fewer than 1 in 100 women within the general population. Men are also affected by BRCA1/BRCA2 mutations, as they are 8 times more likely to be diagnosed with breast cancer than those without the mutation. In addition, BRCA1/BRCA2 mutations increase the risk for high-grade prostate cancer and pancreatic cancer. Mutated BRCA1/BRCA2 genes are inherited in an autosomal-dominant pattern and can come from either parent. Only one copy of the mutated gene in each cell is sufficient to increase the risk of developing cancer. Each child of a parent who carries a BRCA1/BRCA2 mutation has a 50% chance (or a 1 in 2 chance) of inheriting the same gene mutation (CDC, 2021a; NCI, 2020a).
Metastases denote the secondary growth of the primary cancer cell type in another organ. Metastatic disease occurs after a cancer cell detaches from the original tumor site, invades local tissue, and migrates to a distant organ through the bloodstream and/or lymphatic system. Over time, the cell replicates in the new area, creating a secondary tumor site. While each cancer type has its unique spread pattern, the four most common locations that many cancers metastasize to include the liver, lung, bone, and central nervous system. A common misconception among patients with metastatic cancer is that they have developed a second cancer type, so vigilant patient and family education is required to encourage understanding of the distinction (Nettina, 2019; Olsen et al., 2019; Yarbro et al., 2018).
The Role of Oncology Nurses
The role of oncology nurses continues to evolve alongside the advancing treatment landscape. Oncology nurses provide direct patient care, symptom management, palliative care, and hospice/end-of-life care. In addition, they serve as care coordinators, patient navigators, education experts, and cancer survivorship leaders. The scope of practice, responsibilities, and subspecialty training is equally broad. Some work in critical care settings such as intensive care units (ICUs) or bone marrow transplantation; others practice in outpatient infusion centers or radiation departments. Many work in the community, focusing on cancer prevention, screening, and early detection. Practice settings vary between academic and community hospitals, infusion clinics, private medical offices, radiation treatment facilities, and home health care. Over the last decade, there has been a shift in treatment delivery to outpatient settings, reserving inpatient care for surgical needs, higher-level acuity, and oncologic emergencies. As treatments have become increasingly complex, so has the collaborative relationship between nurses and physician/provider teams. Comprehensive, efficient, and safe cancer care is grounded in a strong partnership and clear communication between team members (Neuss et al., 2017; Olsen et al., 2019).
Oncology nurses are tasked with complex responsibilities, such as managing a patient's disease symptoms and anticipating the side effects of various therapies. They must establish a thorough understanding of cancer biology, symptoms, and treatments to ensure they are well-equipped to educate patients about their disease and treatment-related side effects. Furthermore, they need to assess patients' and families' understanding and tailor education accordingly. Patients are often unable to retain all the information presented by their healthcare provider and feel confused, overwhelmed, and panicked following a cancer diagnosis. Oncology nurses answer questions and clarify misconceptions about the disease, prognosis, and treatment options. Skilled nurses understand their role in advocacy for each patient, and they utilize their position to communicate with oncologists on a patient's behalf. A cancer diagnosis is a stressful, life-altering event accompanied by substantial physical, psychological, and social implications. Cancer impacts the entire family, causing fear, uncertainty, anxiety, and grief. Patients may feel powerless, hopeless, and overwhelmed. A comprehensive nursing assessment evaluates for a history of depression, anxiety, or other mental health conditions; these histories increase the risk of developing depression or anxiety in response to cancer. Ongoing assessments for current symptoms of depression or anxiety are essential throughout the disease trajectory. Nurses monitor for signs of emotional distress, poor coping, or other psychological difficulties and organize referrals to specialists, such as dieticians, social workers, physical therapists, counselors, and support groups. Ensuring patients are emotionally healthy is as important as assessing their understanding of the diagnosis and treatment plan (Greer et al., 2020; Nettina, 2019; Neuss et al., 2017).
Nurses are often the first line of contact and communication with patients; thus, exceptional triage skills are required to divert oncologic emergencies and ensure immediate access to care. As cancer care has become increasingly subspecialized, nurses have unique opportunities to cultivate expertise in specific areas of focus, such as breast cancer or gastrointestinal cancers. Thus, it is difficult to define and capture all the roles and responsibilities of an oncology nurse comprehensively, given the wide range of opportunities and high variability between practice settings and patient populations. Nevertheless, many oncology nurses describe a deeply intrinsic meaning and reward attained via their work in cancer care, which varies across environments, cultures, and experiences (Olsen et al., 2019; Yarbro et al., 2018).
The Oncology Nursing Society (ONS) has developed oncology nurse generalist competencies, outlining the basic knowledge and abilities required to practice proficiently and safely. These standards and competencies collectively help safeguard patients and promote high-quality care. Oncology nurses care for a high-risk and complex patient population, serving as integral members of interdisciplinary care teams. The ONS Oncology Nurse Generalist Competencies (2016) support novice oncology nurses' successful transition into entry-level practice and form a firm foundation for daily clinical practice, professional development, and career advancement. The document outlines specific competencies within core areas of teamwork, professional development, evidence-based clinical care, financial toxicity, and quality metrics. In 2008, the American Society of Clinical Oncology (ASCO) and the ONS collaborated to create safety standards for administering antineoplastic agents. The ASCO/ONS Chemotherapy Administration Safety Standards were last revised in 2017 and address oncology practice-related issues and nursing benchmarks to safeguard patient care. These apply across settings and patient populations and serve as the gold standard for oncology nursing practice (Neuss et al., 2017). An oncology nurse's scope of practice is further defined and regulated by state laws and the nursing practice act (Neuss et al., 2017; ONS, 2016).
A Nurse's Role in the Early Detection and Prevention of Cancer
Risk and Protective Factors
While the definitive cause of cancer is not entirely understood, and many people develop the disease without any identifiable causes, numerous factors are known to increase the risk. Some theories postulate that cancer may occasionally occur from the spontaneous transformation of cellular processes and DNA alterations, but most theories blame carcinogens. Carcinogens are substances, radiation, or exposures that damage the cellular genetic material throughout a person's lifetime, resulting in cancer formation. Examples of carcinogens include tobacco, tanning beds, diesel exhaust, and ultraviolet (UV) radiation. Risk factors are categorized as modifiable and or non-modifiable based on the individual's ability to alter the risk. Age is the clearest risk factor for cancer, as cancer incidence rises alongside age. Other non-modifiable risk factors include family history, genetics, and most chemical and radiation exposures. Modifiable risk factors include diets high in fat, obesity, sedentary lifestyles, tobacco use, excessive alcohol intake, and sunlight exposure. Altering modifiable lifestyle risk factors can serve as a protective factor to lower a person's risk of cancer, such as maintaining a healthy weight, smoking cessation, engaging in regular physical activity, and practicing sun safety to limit harmful UV exposure. Research demonstrates that additional cancer risk factors include chronic inflammation and hormones. In addition, some infections and viruses are associated with an increased risk of cancer. Hepatitis B and C are linked to hepatocellular cancer, human papillomavirus (HPV) can cause cervical cancer, and Epstein-Barr virus is associated with Hodgkin lymphoma. People in low- and middle-income countries are at increased risk for developing cancer through chronic infections. Primary and secondary prevention strategies decrease the morbidity and mortality of cancer (NCI, 2019; Olsen et al., 2019; Yarbro et al., 2018).
Primary Prevention
Primary prevention focuses on actions to inhibit cancer from developing. Since many cancer deaths are preventable, primary prevention involves minimizing harmful exposures and reducing or omitting unhealthy lifestyle behaviors. According to the World Health Organization (WHO, 2022), cancer prevention offers the most cost-effective long-term strategy for cancer control worldwide. Research demonstrates that at least 42% of newly diagnosed cancers in the US are potentially avoidable, as they are directly correlated with tobacco use, obesity, physical inactivity, poor nutrition, alcohol use, and other modifiable behaviors. Tobacco is the single most significant cause of cancer-related deaths and is linked to more than 480,000 deaths annually, 41,000 of which are related to secondhand smoke. Cigarette smoking causes about 1 of every 5 deaths in the US each year, and the life expectancy for smokers is at least 10 years shorter than for nonsmokers. Tobacco use causes cancers throughout the body, including lung, bladder, head and neck, kidney, cervix, liver, and pancreas. Skin cancers are primarily due to excessive sun exposure without proper protection and indoor tanning beds. Prevention strategies include sunscreen, lightweight clothing, and hats to shield from direct exposure; reducing sunlight exposure during peak hours of the day when the ultraviolet rays are the strongest; and avoiding tanning beds altogether (ACS, 2022a; CDC, 2020; Nettina, 2019; Olsen et al., 2019).
Cancers related to hepatitis and HPV can be prevented through behavioral and lifestyle changes, such as practicing safe sex and getting vaccinated. According to the CDC (2021c), 85% of people will get an HPV infection in their lifetime, and approximately 13 million people become infected each year. HPV can cause cancers of the cervix, vagina, vulva, oropharynx (throat, tonsils, tongue), penis, and anus. Infections with high-risk HPV subtypes that cause most HPV-related cancers have dropped by nearly 90% due to the HPV vaccination. Cervical cancers linked to HPV have declined by 40% among vaccinated women. The HPV vaccine is highly effective and is recommended for all children (boys and girls) ages 11-12 years. Thus far, more than 135 million doses have been distributed across the US (CDC, 2021c).
Secondary Cancer Prevention
Cancer burden can also be reduced through early detection and treatment. Secondary cancer prevention involves screening to identify cancer before symptoms develop, while the disease is treatable or potentially curable. The goal of screening is to reduce morbidity and increase survival. Screening helps detect high-risk patients who require increased surveillance compared to the general population. Alternatively, high-risk patients may undergo invasive interventions such as prophylactic mastectomy. Preventive surgeries can diminish the lifetime risk of breast or ovarian cancer. Screening colonoscopies promote the early detection and removal of precancerous polyps in the colon before the cells mutate into invasive cancer. Other examples of standard screening tests include sigmoidoscopy, fecal occult blood testing (FOBT), digital rectal examination (DRE), mammography, Papanicolaou testing (Pap smear), and prostate-specific antigen (PSA) testing. In addition, institutions increasingly offer low-dose spiral computed tomography (CT) scans to detect curable stage I lung cancer in patients who meet the designated criteria (e.g., age and tobacco use history). The decision to perform routine screening tests is based on national guidelines, which determine whether the tests are adequate to detect a potentially curable cancer in an otherwise asymptomatic person and are cost-effective. In the formation of screening guidelines, several factors are considered, such as age, sex, family history, ethnicity/race, and iatrogenic factors (e.g., prior radiation therapy or diethylstilbestrol [DES] exposure). While screening is an effective secondary cancer prevention strategy, not all cancers can be detected early. For example, there are no effective screening tests for ovarian and pancreatic cancer, so most are diagnosed at advanced stages when symptoms present. Screening tests are imperfect and may not identify cancer in all patients. Each test has a risk-benefit ratio that must be considered and explained to the patient. Oncology nurses play a vital role in assessing individual risk factors, monitoring for symptoms, and educating patients on the importance of lifestyle modifications and the benefits and limitations of screening. Fundamental training and teaching points are outlined in Table 1, as adapted from the ACS Prevention and Early Detection Guidelines (ACS, 2021; Yarbro et al., 2018).
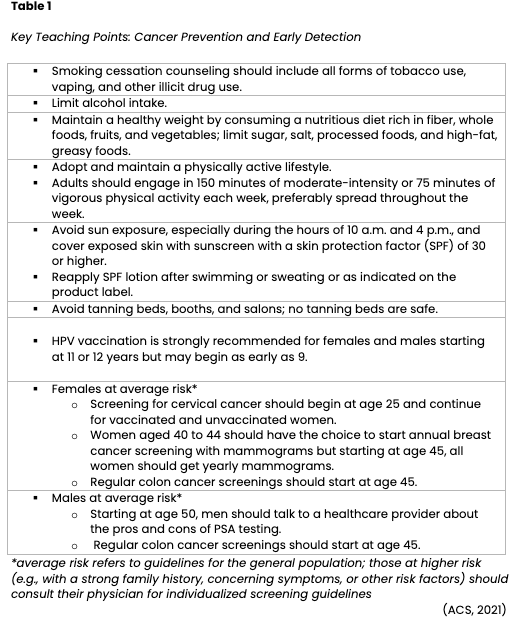 Refer to the Cancer Prevention and Earning Detection NursingCE course for more detailed information on cancer screening.
Refer to the Cancer Prevention and Earning Detection NursingCE course for more detailed information on cancer screening.
The Relationship between Obesity and Cancer
Obesity is a risk factor for cancer development, progression, and recurrence. According to the CDC (2021b), obesity is linked to at least 13 types of cancer, which comprise 40% of all cancers diagnosed in the US each year (see Table 2). Measured as a body mass index (BMI) of 30 or higher, obesity is defined as excess fat accumulation in relation to height. Despite its negative impact on health, obesity rates are still expected to rise substantially over the next several decades. Nearly 20% of all cancers diagnosed in the US are related to excess body weight, physical inactivity, and poor nutrition; aside from tobacco use, these are the three most crucial cancer risk factors that can be modified (ACS, 2020a). 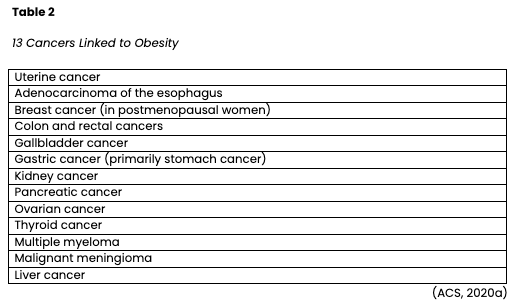
A primary example of the relationship between cancer and obesity is endometrial (uterine) cancer. Endometrial cancer is the most common gynecologic malignancy, with an estimated 66,570 new cases and 12,940 deaths in the US in 2021 (Surveillance Epidemiology and End Results Program, 2021). Historically recognized as a disease of postmenopausal women, endometrial cancer has become more prevalent in the younger, premenopausal population. The increased incidence is attributed to the global obesity epidemic and associated metabolic disorders. Obesity is cited as a causative factor for approximately 80% of endometrial cancers worldwide. Overweight and obese women are up to 4 times as likely to develop endometrial cancer than normal-weight women, and morbidly obese women are nearly 7 times more likely to develop cancer. The relationship is primarily driven by an overproduction of estrogen carried in excess adipose tissue. Additional factors such as insulin resistance, increased bioavailability of steroid hormones, and inflammation also contribute to cancer development. Obesity leads to poorer long-term health outcomes and is also found to impact the treatment course negatively. Despite a clear understanding of the relationship between obesity and endometrial cancer, mortality from obesity-driven comorbidities continues to rise. Furthermore, obese women with endometrial cancer are more likely to die from other obesity-related diseases than endometrial cancer. These conditions are some of the leading causes of preventable death, including heart disease, stroke, and type II diabetes (Kokts-Porietis et al., 2021; Sagnic, 2021; Smrz et al., 2021 ).
Cancer Signs and Symptoms
The presenting signs and symptoms of cancer can vary widely depending on the type of cancer. A patient may be asymptomatic at diagnosis, or signs may be vague and nonspecific, prolonging the time from symptom presentation until diagnosis. Some cancers, such as leukemia and lymphoma, often exhibit a cluster of signs that suggest the diagnosis, such as constitutional symptoms of weight loss, night sweats, lymphadenopathy (enlarged lymph nodes), and excessive fatigue. The differential diagnosis for cancer is extensive, with many symptoms overlapping with those of common benign illnesses, such as viruses or tick-borne diseases. However, certain warning signs may indicate an underlying malignant process and should be evaluated. Ominous symptoms include rectal bleeding, vaginal bleeding in postmenopausal women, a lump in the breast, abdominal bloating or distension that does not resolve, or unexplained pain. Other signs may include hoarseness, productive coughing with hemoptysis (blood-streaked sputum or coughing up blood clots), changes in bladder or bowel habits, and unusual bleeding or bruising. Cancer may present in various ways, so a comprehensive medical history and physical assessment are critical (ACS, 2020b; Yarbro et al., 2018).
Overview of Cancer Diagnosis and Staging
Patients undergo a series of tests to establish the origin and extent of their cancer. A tissue sample, bone marrow aspiration, or cytology specimen is necessary to determine the cell type, grade, and unique features. A tissue sample (or biopsy) is most commonly performed when evaluating solid tumors such as breast or pancreatic cancers. Patients may undergo a fine-needle aspiration (FNA), a procedure in which cancer cells are aspirated from the tumor using a needle and syringe. However, an FNA cannot distinguish invasive from noninvasive cancer, and negative results do not entirely rule out malignancy; thus, a core-needle biopsy is commonly performed. This technique utilizes a large-bore needle placed directly into the tumor, usually through ultrasound or computed tomography (CT) guidance, to retrieve a small piece of cancer. A core biopsy typically provides enough tissue to diagnose most cancer types and is often performed in outpatient settings. At other times, tumor samples may be retrieved through surgical intervention such as lymph node excision or open biopsies performed in an operating room when patients are under anesthesia (Longo, 2019; Yarbro et al., 2018).
A bone marrow biopsy is more commonly performed for hematologic malignancies such as leukemia or multiple myeloma. A small sample of the bone marrow is extracted for testing, most commonly from the hip bone. A wide needle is inserted into the hip bone and rotated to remove a sample of the bone. Cytology examines small clusters of cells in body fluids. It differs from tissue sampling in that cytology samples consist of a suspension of cells instead of tissue. Cytology examinations can be performed on various fluid collections such as cerebrospinal fluid (CSF), pleural fluid, or ascites. The fluid is extracted using a long, thin needle. Cytology can also be performed by scraping or brushing cells from the tissue or organ being tested—like the cervix, esophagus, or bronchi—using a small brushing instrument (e.g., Pap smear). The collection procedure varies depending on the location of the specimen. For example, a paracentesis is performed to collect fluid from the abdominal cavity, whereas a thoracentesis extracts cells from the pleura (Longo, 2019; Yarbro et al., 2018)
Tumor grading, also called histologic grading, measures cancer cells' appearance compared to that of healthy cells under a microscope. It also describes how quickly the cells may grow and spread. This information is vital to determine the patient's prognosis and treatment plan. Tumor grading systems differ for each cancer type but are primarily based on cellular differentiation and ranked from low-grade (Grade 1) to high-grade (Grade 3). Low-grade cancers look similar to healthy tissue, are the least aggressive, and grow and spread slowly. High-grade cancers are poorly differentiated or undifferentiated, as cells do not resemble healthy tissue and are the most aggressive. Poorly differentiated cancers typically have the worst prognosis. Specialized staining is performed to look for specific markers or proteins in a tumor. Certain types of cancer have unique proteins (antibodies) on their cell's surface, and immunohistochemical (IHC) staining helps identify whether these antibodies are present; this information helps develop the treatment regimen. For instance, IHC testing for breast cancer evaluates whether the tumor overexpresses two hormones, estrogen receptor (ER) and progesterone receptor (PR). If the tumor is positive for ER and/or PR, growth is fueled by hormones, and the patient will likely benefit from hormone-blocking therapies. If these markers are negative, the patient will not benefit from hormonal treatments (Longo, 2019; Yarbro et al., 2018).
Flow cytometry evaluates cells extracted from the bone marrow, lymph nodes, or bloodstream for antibody receptors; measures the amount of DNA in cancer cells; and looks for other biomarkers to guide treatment. Genetic testing is increasingly utilized in cancer care to identify targetable gene mutations and abnormal chromosomes as components of personalized medicine. Scientific advancements have led to the inception of gene profiling and cytogenetic testing as foundational components of cancer diagnosis and treatment planning. Fluorescent in situ hybridization (FISH) testing uses fluorescent dyes linked to pieces of DNA that attach only to specific parts of particular chromosomes. Identifying these chromosomal alterations is vital in determining whether targeted drugs might be effective. For example, FISH testing can reveal if there are too many copies (amplification) of the human epidermal growth factor receptor 2 (HER2) gene, which creates a protein found on the surface of all breast calls. Overexpression of HER2 is a marker for cancer growth and is likely to benefit from anti-HER2-based treatment, such as trastuzumab (Herceptin; Longo, 2019; Olsen et al., 2019; Yarbro et al., 2018).
Radiology imaging such as CT scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans are other vital components of the cancer staging workup. Additional radiographic testing may be necessary depending on the type of cancer suspected. Accurate staging provides a collection of data that informs decision-making about treatment and guides treatment outcomes for each type of cancer. The American Joint Committee on Cancer (AJCC) has developed a simple classification system that can be applied to nearly all tumor types called the tumor-node-metastasis (TNM) staging system. The TNM is preferred for most solid tumors and is a numeric assessment of the tumor size (T), presence or absence of regional lymph node involvement (N), and presence or absence of distant metastasis (M). However, there is no single standard evaluation tool for all cancers. Hematologic malignancies are staged differently than solid tumors. For example, Hodgkin and Non-Hodgkin lymphomas are staged with the Ann Arbor Classification, whereas multiple myeloma staging relies on the International Staging System (ISS) and Revised ISS (R-ISS). In general, the components of the staging workup depend on the patient, tumor type, symptoms, pathophysiology, and classic spread pattern of each cancer (Olsen et al., 2019; Yarbro et al., 2018).
Tumor Markers
Tumor markers are substances, or proteins, made in higher quantities by cancer cells than normal cells. In some patients, tumor markers are detected and measured in the bloodstream or urine. They can provide information about the cancer, such as how aggressive it is, whether it is responding to treatment, or if it is growing. However, they are nonspecific metrics and can sometimes increase due to benign causes, such as infection or inflammation. Furthermore, not all cancers produce measurable tumor markers. Some common tumor markers include PSA, carcinoembryonic antigen (CEA), cancer antigen 125 (CA 125), and cancer antigen 27-29 (CA 27-29). Each tumor marker is specific to a type of disease process. For instance, PSA relates to prostate cancer; CEA is related to cancers of the rectum, colon, pancreas, and breast; CA-125 is linked to ovarian cancer; and CA 27-29 is associated with breast cancer (NCI, 2021a; Yarbro et al., 2018).
Treatment Modalities
There are four main goals of cancer therapy: prevention, cure, control, and palliation. While prevention focuses on inhibiting cancer development, cure denotes treatment to eradicate the disease. Control refers to extending the patient's life when a cure is unlikely or impossible by preventing the growth of new cancer cells and reducing the size and impact of an existing disease. Finally, palliation centers on comfort when cure and control of the disease cannot be achieved. Oncology nurses should engage in ongoing discussions with patients regarding their care goals and whether their goals are being achieved. The primary categories of cancer treatment include surgery, radiation, chemotherapy, and biological therapies, as shown in Figure 1 (Nettina, 2019; Yarbro et al., 2018).
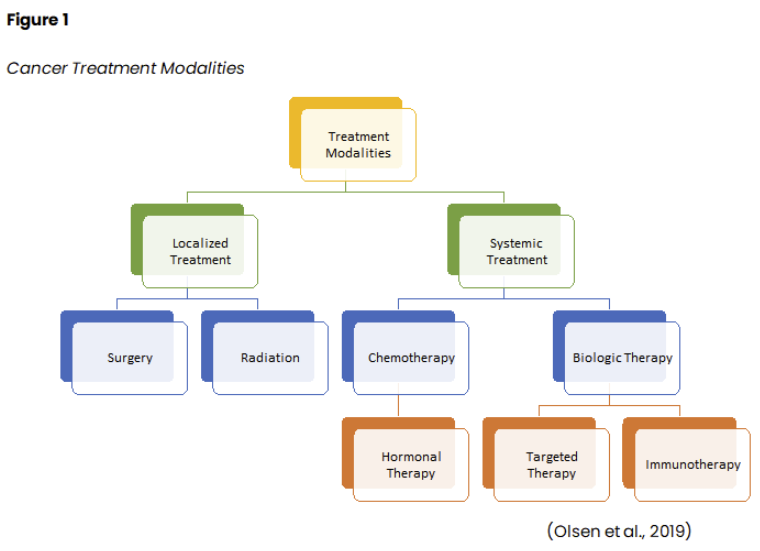
Often, patients receive a combination of treatment modalities throughout the disease trajectory. Scientific advancements and treatment breakthroughs have revolutionized how cancer is managed, leading to innovative fields such as precision (or personalized) medicine, targeted therapies, and immunotherapy. Precision medicine uses the genomic profiling of a patient's tumor to identify genetic mutations unique to the tumor. Targeted therapies block the growth and spread of cancer by interfering with specific genes, proteins, and/or blood vessels that allow cancer cells to replicate, grow, and spread. Immune-based treatments assist the immune system in identifying cancer cells and attacking them as it would for bacteria or other foreign pathogens. Radiation therapy is a localized treatment that has become more precise and effective over time; however, it still carries many associated side effects and toxicities. Surgery is not indicated for all cancers, but clinical research has helped better define the parameters and indications for surgical intervention and the ideal time for patients to undergo surgery (Yarbro et al., 2018). The National Comprehensive Cancer Network (NCCN, 2022) is an alliance of leading cancer centers and world-renowned experts devoted to cancer care, research, and education. The NCCN provides evidence-based treatment guidelines according to cancer type, pathology, genetics, staging, inheritance patterns, and several other specific features through rigorous clinical trial research, data compiled across institutions, and annual expert panel reviews. The guidelines are widely utilized in cancer care and guide medical decision-making throughout a patient's disease trajectory (NCCN, 2022).
Surgical Treatment
Surgical management of cancer is commonly performed for tumors confined locally or regionally. For cancers that grow slowly and remain confined, surgery has a greater chance of providing control and curing the disease. Surgery is performed with curative intent when the goal is to remove all or a significant portion of the primary tumor. It may be the only treatment necessary or performed before or after other treatment modalities. There are other times when surgery is used in a palliative setting to reduce and alleviate distressing cancer symptoms. The role of surgery can be categorized into preventive, primary surgery, cytoreductive surgery, salvage treatment, palliative treatment, and reconstructive (Yarbro et al., 2018). Table 3 outlines the various types of surgical intervention for cancer treatment.

Surgery may be combined with other treatment modalities, such as preoperative chemotherapy (neoadjuvant therapy), intraoperative chemotherapy, radiation therapy, or postoperative chemotherapy (adjuvant therapy). The type and severity of side effects or surgical complications depend on the specific treatment modality and comorbid conditions. For example, some chemotherapy agents can delay wound healing, whereas others may impact cardiac function, imposing surgical risks and complications. In addition, patients with diabetes or underlying cardiac disease are at increased risk for infection or fluid overload. The nurse's role extends throughout the surgical continuum, from the preoperative to the postoperative period. Nursing responsibilities are broad and may include obtaining pertinent medical history, allergies, and reviewing surgical consent. In addition, nurses are responsible for pre-surgical counseling and teaching, such as preparing for surgery, infection control measures, expectations during the surgery, and proper skin care (Nettina, 2019; Yarbro et al., 2018).
Nurses manage postoperative pain, nausea, and adverse reactions to anesthesia. They perform interventions to decrease the incidence and severity of surgical complications by promoting airway clearance, monitoring surgical sites, and performing aseptic wound care. Postoperative nursing care focuses on pulmonary rehabilitation, promoting coughing, deep breathing, and incentive spirometry to prevent pneumonia and lung infections. Nurses teach splinting of the incision with coughing, sneezing, or other movements to reduce pain and avoid wound dehiscence. They are responsible for ensuring and advocating for adequate pain control and administering pain medications. Nurses reposition patients in bed every 2 hours to prevent pressure injuries and skin breakdown. They monitor closely for signs and symptoms of infection, such as fevers, redness, drainage, swelling, or warmth at the incision site, and promptly report suspicious changes or concerns to the provider (Nettina, 2019; Yarbro et al., 2018).
Cancer patients are hypercoagulable or at increased risk for venous thromboembolism (VTE). Abnormalities within the coagulation cascade, fibrinolytic pathways, and/or platelet function contribute to hypercoagulability and subsequent blood clot formation. Hypercoagulability associated with cancer is multifactorial, but research suggests it is primarily due to tissue damage that triggers the coagulation cascade and increased inflammatory markers in circulation. Surgery compounds this pre-existing risk, increasing the propensity to develop a blood clot in the postoperative period (Longo, 2019). Nurses must ensure patients receive appropriate VTE prophylaxis after surgery to mitigate their VTE risk, such as sequential compression devices (SCDs), compression stockings, and anticoagulation therapy. Early mobility and adequate hydration are encouraged to reduce VTE risk postoperatively. Nurses are responsible for monitoring any signs of a blood clot, such as unilateral leg swelling, calf or knee discomfort, redness, warmth, shortness of breath, hypoxemia, or tachycardia. These symptoms must be promptly reported to the provider, as they can quickly progress and lead to fatal outcomes. Surgical nurses often manage many invasive tubes, drains, and catheters in the postoperative period. Cautious assessment and monitoring of each site are crucial to identify acute signs of infection or other complications. Surgical complications may include infection, bleeding, thrombosis, bowel obstruction or ileus, acute respiratory distress syndrome, aspiration pneumonia, and cardiac dysfunction (Longo, 2019; Yarbro et al., 2018).
Surgical oncology nurses prepare patients for discharge, ensure all needs are addressed, teach them how to care for surgical wounds, and coordinate follow-up visits. Patients and their caregivers must understand how to administer prescribed medications, dosing schedules, and expected side effects and recognize signs of infection. Nutrition is critical postoperatively, as it has implications for wound healing, infection control, and overall prognosis. Protein and caloric malnutrition are common complications in patients who have undergone prior cancer therapy or are immunocompromised. Inadequate nutrition can increase the risk of wound dehiscence, sepsis, and prolonged hospitalizations. In addition, the management of any temporary or permanent lines, catheters, or body alterations, such as ostomy care, must be reviewed. Communication with home health agencies is imperative to convey postoperative instructions and ensure a seamless transition without disruption in care. Patients may experience a significant emotional component when undergoing surgery, and some patients first learn of their cancer diagnosis during their postoperative recovery. Oncology nurses are uniquely positioned to defuse distressing events, provide emotional support, and reinforce education (Yarbro et al., 2018).
Radiation Therapy
Radiation therapy (or radiotherapy) has dramatically evolved since Cobalt therapy in the 1950s. Computers now dictate radiation treatment planning and dose optimization. Radiation plays a central role for many cancer types, with more than half of cancer patients receiving radiotherapy at some point during their treatment. Radiotherapy delivers high doses of radiation to a tumor while limiting exposure to surrounding healthy tissue. Radiation causes biological changes in cellular DNA, causing cell death over days, weeks, and months. All healthy cells and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed. However, healthy cells can repair themselves and remain functional. Rapidly dividing cancers, such as lymphomas and squamous cells of the head and neck, tend to be more sensitive to the effects of radiation than cancers that divide more slowly, such as sarcoma. The goal is to eradicate the tumor, palliate symptoms, improve quality of life, and/or prolong survival with as minimal morbidity as possible (NCCN, 2021; Yarbro et al., 2018).
Radiation may be the only treatment required, or it may follow another treatment such as surgery. Some patients receive chemotherapy concurrently as the chemotherapy acts as a radiosensitizer, making the cancer cells more vulnerable to radiation. Patients who undergo surgery to remove a cancerous tumor may need radiation to the affected area after surgery (adjuvant radiation) to ensure all cancer cells are eradicated. Palliative radiation is given to alleviate pain and reduce symptom burden. For patients with primary spinal cord tumors or metastatic disease to the spine, palliative radiation is given to ease discomfort or relieve neuropathy and neurologic paresthesia from tumor compression on the nerves and spinal cord. Palliative radiation for brain tumors can help improve headaches, nausea, vomiting, or visual disturbances. The total dose of radiation is hyper-fractionated, which means it is administered in smaller divided doses, or fractions, rather than all at once. Hyper-fractionation gives healthy cells a chance to recover between dosing. While there are a variety of treatment plans, patients usually receive daily treatments over several weeks with weekend breaks. The weekend break allows for the repair and repopulation of surrounding healthy tissue to reduce toxicity and side effects while still targeting the disease. The total number of fractions administered depends on various factors such as the tumor size, location, cancer type, the patient's overall health, performance status, and goals of therapy. Radiation can be delivered externally or internally, and some patients may receive both. Table 4 compares external and internal radiation therapies (Nettina, 2019; Yarbro et al., 2018).
Before radiation begins, patients undergo a simulation procedure using CT or MRI imaging to guide the precise location of the radiation beams. A pinhead-sized tattoo is typically applied to the skin and used as a target. When the simulation plan is completed, the radiation oncologist reviews all the data, and multiple validations of the treatment plan are performed to ensure high-quality and appropriate treatment. While some forms of radiation therapy are administered in an inpatient setting, most are delivered on an outpatient basis in hospital-based radiation departments or free-standing radiation facilities. Once treatment begins, daily treatments average less than 15 minutes in duration each, and most people can schedule radiation treatments around their routine (Nettina, 2019). 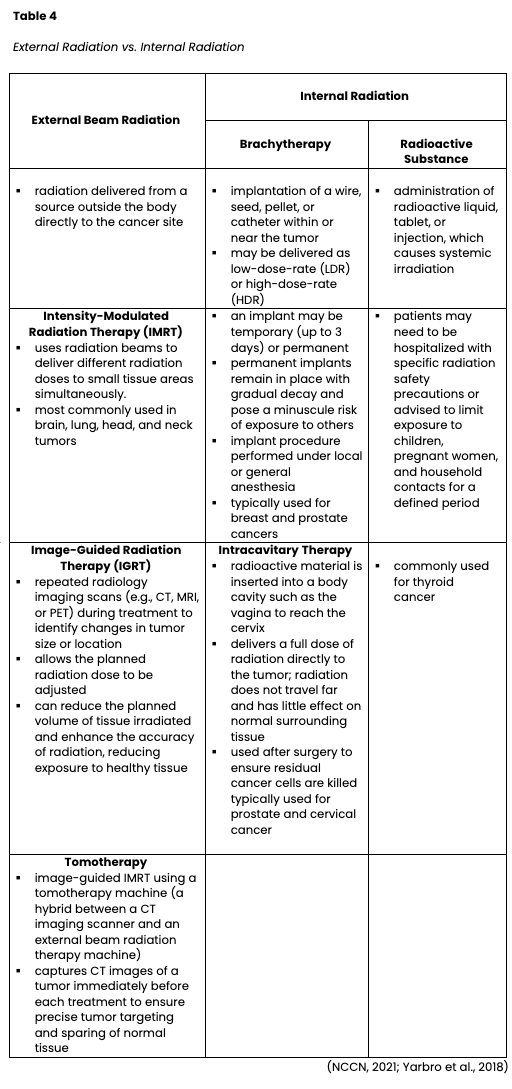
LDR brachytherapy typically requires hospitalization for several days, as the patient remains in an isolated, radiation-safe room to prevent exposure to others. Some LDR treatments require the patient to be confined to a bed to avoid the dislodgment of the radioactive applicator for 1-3 days. LDR brachytherapy is used for prostate cancer, oral cancers, and cervical cancer. HDR brachytherapy has distinct differences from LDR and is generally performed on an outpatient basis. Each treatment lasts a few minutes, and hospitalization and bed rest are not indicated. Staff, visitors, and family members are not exposed to radiation. The treatment is delivered in a radiation-shielded room to protect others from exposure, and patients are not considered "radioactive" after each treatment. They can safely go about their regular routines and lifestyles without potentially exposing others. HDR brachytherapy is used to treat many cancers, such as lung, breast, and esophageal cancers (NCCN, 2021).
Two unique types of radiation therapy include intraoperative radiation therapy and stereotactic radiation or radiosurgery. Intraoperative radiotherapy is a technique performed in the operating room at the time of surgery. While the tumor bed is exposed, a large dose of radiation is delivered to the area. Reserved for tumors that are not wholly operable or have a high risk of recurrence, this technique is most commonly performed with CRC and gynecologic malignancies. Radiosurgery is widely used for the treatment of brain tumors. The radiation beam is delivered precisely to the tumor, sparing the surrounding healthy brain tissue. This can be administered through a procedure called gamma knife, during which the patient's head is placed into a frame and secured with screws, while specialized scanning technology allows for precise and accurate delivery of radiation to the tumor (Yarbro et al., 2018).
Radiation Safety
Federal and state regulatory agencies govern the use of radioactive substances and have established strict guidelines for safety, exposure, and maximum permissible doses for radiation oncology nurses. Nurses caring for radiotherapy patients must undergo extensive training in the critical components of radiation safety and wear badges that track their radiation exposure. To protect themselves from exposure, they need to remain vigilant about the three radiation principles of time, distance, and shielding. Time refers to minimizing the time spent near the source. Nurses are taught to cluster care activities to reduce time spent in direct contact and maximize their distance from the source. Shielding refers to protective barriers between the radiation source and the nurse whenever possible. For example, if a nurse attends to patient needs at the bedside, aside from clustering activities, specialized personal protective equipment is worn to shield them from the radioactive source. The nurse is also responsible for educating the patient and their family members on appropriate safety measures, limiting family members' contact with the patient, and providing emotional and psychological support to the patient, who often feels lonely and isolated. For the subset of patients who are radioactive due to seeds or implanted devices when discharged into the community, healthcare providers and radiation oncology nurses play critical roles in educating them about the risk their therapy can pose to others. Before discharge, the patient and their caregiver should fully understand the necessary safety and risk-reducing precautions. Patients are encouraged to avoid young children and pregnant women until the radioactivity decays to safe levels. Policies and regulations protect radiation oncology nurses and other radiation personnel who may be pregnant. In general, policies discourage pregnant personnel from caring for patients receiving radiation. Nurses are advised to notify their supervisor immediately if they are pregnant or trying to become pregnant (NCCN, 2021; Nettina, 2019; Yarbro et al., 2018).
Nursing Implications in Radiation Therapy
The ONS offers an international web-based continuing education course, the ONS/ONCC Radiation Therapy Certificate Course , which oncology nurses practicing in radiation specialties are strongly encouraged to complete. While teaching is a primary responsibility, as safety is of utmost concern for this patient population, nurses also play a vital role in symptom management (ONS, 2019).
Acute Effects of Radiation
Radiation can produce acute and latent side effects. Since radiation therapy is a localized treatment, side effects vary based on the targeted location and the dose. The most common generalized side effects include skin reactions (external skin or mucous membranes) and fatigue. The acute effects of radiation are usually transient, begin about 2 weeks after starting treatment, and subside within 2 weeks of completing treatment. Radiation affects cells with rapid renewal characteristics that quickly turnover, such as the skin, mucous membranes, and bone marrow. Other factors such as age, nutritional status, and prior or concurrent chemotherapy also impact the severity of acute symptoms. Fatigue and anorexia are the most common generalized effects. Other acute effects are site-specific and may include local inflammation (e.g., gastritis, esophagitis, colitis), alopecia, lymphedema, and sexual dysfunction (Nettina, 2019; ONS, 2019; Palmer et al., 2021).
Radiation targeting the GI tract, such as the stomach, colon, or rectum, can cause nausea, vomiting, diarrhea, painful defecation, dehydration, weight loss, and skin breakdown. If the anus is affected, patients may experience fecal incontinence, rectal bleeding, or hemorrhoids. Head and neck radiation can cause numerous complications such as oral ulceration (mucositis), esophageal ulceration (stomatitis), painful swallowing (dysphagia), and dry mouth (xerostomia). These patients often require a feeding tube to ensure adequate nutrition and prevent cachexia. Nutrition is a core component of treatment and influences patients' ability to tolerate therapy. Therefore, many physicians recommend percutaneous endoscopic gastrostomy (PEG) tube placement before treatment begins. Radiation to the breast or chest wall can impact the heart and lungs, leading to late effects such as cardiotoxicity (damage to the heart function or muscle) or pulmonary fibrosis (scarring of the lungs). Radiation to the cervix and vagina can lead to vaginal atrophy, inducing symptoms of vaginal dryness, irritation, scarring, and sexual dysfunction. Radiation fields that affect the bladder can cause cystitis (inflammation of the bladder), leading to dysuria (painful urination), hematuria (blood in the urine), urinary incontinence, and loss of pelvic floor muscular strength. Radiation near the spine can cause bone marrow suppression, such as neutropenia, anemia, and thrombocytopenia (ONS, 2019; Palmer et al., 2021; Yarbro et al., 2018).
Skin Care
Skin care is critical for patients undergoing radiation, as up to 95% of patients will experience some degree of skin reaction. Radiation nurses are responsible for educating, assessing, and monitoring patients for radiation dermatitis or radiodermatitis. This condition occurs in response to ionizing radiation exposure and is caused by changes to the basal layer of the epidermis and the dermis. Cumulative doses of radiation weaken the skin integrity, depleting stem cells from the basal layer of the epidermis and leading to varying degrees of radiodermatitis. Acute skin reactions generally begin 7 to 14 days after the starting treatment, and the first signs include dryness and slight erythema. As treatment continues, symptoms can progress to bright red erythema, a rash, and desquamation. Patients often describe the skin as feeling similar to a sunburn. Several factors heighten the risk of skin reactions, such as poor nutrition, excess fat tissue causing skin folds in the radiation field, sun exposure, and use of topical irritants. Desquamation is the sloughing of the top layer of the skin and involves two stages—dry and moist. The first stage, dry desquamation , is the peeling of the top layer of skin and becomes increasingly uncomfortable as the underlying nerve endings are exposed to air. It is most common in intertriginous regions where the skin rubs, such as beneath the breast or axillae. Moist desquamation refers to the peeling of the skin with serous fluid leakage and is very uncomfortable for patients. A radiation oncology nurse's role in skin care relating to radiation is imperative and involves comprehensive assessment, along with the timely delivery of evidence-based interventions. Nurses must also clearly convey education and teaching to patients to ensure an adequate understanding of skin care to minimize skin reactions ( Palmer et al., 2021; Yarbro et al., 2018).
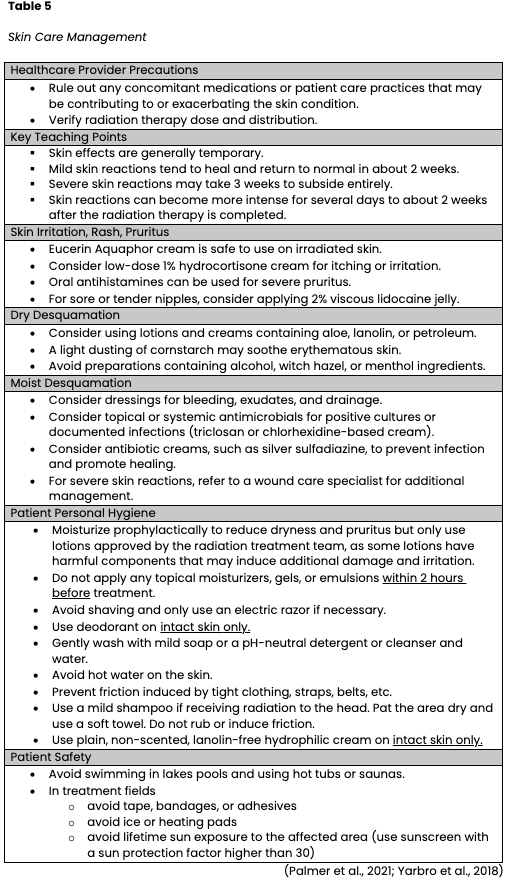
Radiodermatitis can impact a patient's quality of life, cause pain, limit activities, and delay treatment. In severe cases, radiodermatitis can cause treatment cessation. Preventing radiation skin reactions is difficult, particularly for patients with conditions such as inflammatory breast cancer, where intense skin reactions are expected. Therefore, radiation oncology nurses must understand how to monitor, assess, document, grade, and manage radiation dermatitis promptly. Several grading tools are available for evaluating radiodermatitis, and selection can vary across treatment facilities and physician preferences. Some commonly used tools include the Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria, ONS Radiation Therapy Patient Care Record, and the Radiation-Induced Skin Reaction Assessment Scale. The same scale should be used consistently to ensure accurate and reliable assessment and documentation. The management of radiation dermatitis is complex and varied; currently, no gold standard exists for the prevention or control of radiodermatitis. However, expert opinion and consensus have formulated guidelines for radiation oncology nurses. Some of the priority recommendations are outlined in Table 5 (ONS, 2019; Palmer et al., 2021; Yarbro et al., 2018).
Radiation Recall
Radiation recall is a severe skin reaction after certain chemotherapeutic drugs are administered during or soon after radiation treatment. An inflammatory response at a previously irradiated site is triggered mainly by an antineoplastic agent, although the response mechanism is poorly understood. It usually affects the part of the body that receives radiation, especially the skin. The rash appears like a severe sunburn with redness, swelling, and tenderness. In addition, the skin may blister and peel, and discoloration may occur after it heals. Radiation recall can occur weeks, months, or even years after radiation therapy has ended. Treatment generally consists of corticosteroids to reduce inflammation and rarely delayed chemotherapy until the skin heals (ONS, 2019; Yarbro et al., 2018).
Late Effects of Radiation Therapy
Delayed effects appear more than 2 months (in many cases, years) after the exposure. Newer methods of radiation therapy have helped to minimize damage to healthy tissue; however, treatment is directed to the same area each time, and radiation rays sometimes scatter. Tissues and organs near the cancer site can receive small doses of radiation despite efforts to prevent this. Late effects of radiation are based on the location of the radiation treatment site and the dose/duration of treatment. Some late effects include cataracts, permanent hair loss, and neuropsychology problems such as impaired memory or cognition. Others may experience impaired thyroid or adrenal function. Hypothyroidism is a common late effect of radiation therapy to the neck, head, and chest. Radiation-induced heart disease is a complication of radiation to the chest wall, such as in breast cancer or lymphomas, due to the heart's positioning within the radiation field. These patients can develop cardiomyopathy, congestive heart failure, and damage to the heart muscle, inducing an overall decline in cardiac function. Radiation to the posterior chest wall may cause lung complications such as pulmonary fibrosis and interstitial lung disease. Infertility, sterility, and sexual dysfunction are also common delayed and ongoing effects of radiation. Some patients may sustain a decreased range of motion in the treated area due to scarring and loss of tissue elasticity. Vaginal dilators are recommended for patients who have undergone radiation therapy to the vagina. Pelvic floor training exercises (e.g., Kegel exercises) are advised for patients experiencing urinary dysfunction, such as incontinence or loss of sphincter tone, as a means of strengthening the muscles of the pelvic floor. Edema and lymphedema are potential side effects due to the disruption of the lymphatic system. Skin sensitivity with sun exposure to the affected area may persist for life, so patients must be counseled on sun safety practices. Young children who have undergone radiation treatment may exhibit slowed or halted bone growth (ONS 2019; Palmer et al., 2021).
Secondary malignancies are the most severe and dreaded late complication of cancer therapy and comprise about 15% to 20% of all cancer diagnoses. Although most of these cases are linked to chemotherapy exposure, some are related to radiation. Research demonstrates that radiation-induced secondary malignancies are biologically aggressive cancers and histologically different from the primary tumor. The exact mechanism is unknown, but they typically present 5–10 years after radiation therapy for hematologic malignancies and about 10–60 years after for solid tumors (Demoor-Goldschmidt & de Vathaire, 2019; Khanna et al., 2021). According to Palmer and colleagues (2021), secondary malignancies are the leading cause of non-relapse late effects among childhood cancer survivors. In addition, adolescents and young adult cancer survivors are prone to developing second cancers, especially lung cancer. Therefore, patients must be educated on the risk for secondary malignancies to ensure they receive adequate surveillance and screening long-term.
To continue this 2-part learning series, please refer to Oncology Nursing Part 2: Chemotherapy and Oncologic Emergencies
References
American Cancer Society. (2019). Cancer treatment & survivorship facts & figures. https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html
American Cancer Society. (2020a). Diet and physical activity: What's the cancer connection? https://www.cancer.org/cancer/cancer-causes/diet-physical-activity/diet-and-physical-activity.html
American Cancer Society. (2020b). Signs and symptoms of cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/signs-and-symptoms-of-cancer.html
American Cancer Society. (2021). American Cancer Society prevention and early detection guidelines. https://www.cancer.org/healthy/find-cancer-early/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
American Cancer Society. (2022a). Cancer facts & figures 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html
American Cancer Society. (2022b). Cancer facts & figures for African American/Black people 2022-2024 . https://www.cancer.org/research/cancer-facts-statistics/cancer-facts-figures-for-african-americans.html
American Cancer Society. (2022c). Cancer statistics center: 2022 estimates. https://cancerstatisticscenter.cancer.org/
Centers for Disease Control and Prevention. (2020). Tobacco-related mortality. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm
Centers for Disease Control and Prevention. (2021a). BRCA gene mutations. https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/brca_gene_mutations.htm
Centers for Disease Control and Prevention. (2021b). Obesity and cancer. https://www.cdc.gov/cancer/obesity/index.htm
Centers for Disease Control and Prevention. (2021c). Reasons to get HPV vaccine. https://www.cdc.gov/hpv/parents/vaccine/six-reasons.html
Demoor-Goldschmidt, C., & de Vathaire, F. (2019). Review of risk factors of secondary cancers among cancer survivors. British Journal of Radiology, 92 (1093). https://doi.org/10.1259/bjr.20180390
Greer, J. A., Applebaum, A. J., Jacobsen, J. C., Temel , J. S., & Jackson, V. A. (2020). Understanding and addressing the role of coping in palliative care for patients with advanced cancer. Journal of Clinical Oncology, 38 (9). https://doi.org/10.1200/JCO.19.00013
Khanna, L., Prasad, S. R., Yedururi , S., Parameswaran, A. M., Marcal , L. P., Sandrasegaran , K., Tirumani , S. H., Menias , C. O., & Katabathina , V. S. (2021). Second malignancies are radiation therapy: Update on pathogenesis and cross-sectional imaging findings. RadioGraphics , 41 (3). https://doi.org/10.1148/rg.2021200171
Kokts-Porietis, R. L., Elmrayed , S., Brenner, D. R., & Friedenreich , C. M. (2021). Obesity and mortality among endometrial cancer survivors: A systematic review and meta-analysis. Obesity Reviews, 22 (12). https://doi.org/10.1111/obr.13337
Longo, D. L. (2019). Harrison's hematology and oncology (3 rd ed.). McGraw-Hill Education.
National Cancer Institute. (2019). Infectious agents. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents
National Cancer Institute. (2020a). BRCA gene mutations: Cancer risk and genetic testing. https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet
National Cancer Institute. (2020b). Cancer statistics. https://www.cancer.gov/about-cancer/understanding/statistics
National Cancer Institute. (2020c). Cancer disparities. https://www.cancer.gov/about-cancer/understanding/disparities
National Cancer Institute. (2021a). Tumor markers. https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-fact-sheet
National Cancer Institute. (2021b). What is cancer? https://www.cancer.gov/about-cancer/understanding/what-is-cancer
National Comprehensive Cancer Network. (2021). NCCN radiation therapy compendium. https://www.nccn.org/compendia-templates/compendia/radiation-therapycompendium
National Comprehensive Cancer Network. (2022). NCCN guidelines for the treatment of cancer by site . https://www.nccn.org/guidelines/category_1
Nettina, S. M. (2019 ). Lippincott manual of nursing practice (11 th ed.). Wolters Kluwer.
Neuss, M. N., Gilmore, T. R., Belderson, K. M., Billett, A. L., Conti-Kalchik, T., Harvey, B. E., & Polovich, M. (2017). Updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. Oncology Nursing Forum, 44 (1), 31-43. https://doi.org/10.1188/17.ONF.31-43
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and Immunotherapy guidelines and recommendations for practice (1 st Ed.). Oncology Nursing Society.
Oncology Nursing Society. (2016). Oncology nurse generalist competencies . Retrieved from https://www.ons.org/sites/default/files/2017-05/Oncology_Nurse_Generalist_Competencies_2016.pdf
Oncology Nursing Society. (2019). ONS/ONCC radiation therapy certificate course . https://www.ons.org/courses/onsoncc-radiation-therapy-certificate-course
Palmer, J. D., Tsang, D. S., Tinkle, C. L., Olch, A. J., Kremer, L. C. M., Ronchkers, C. M., Gibbs, I. C., & Constine, L. S. (2021). Late effects of radiation therapy in pediatric patients and survivorship. Pediatric Blood & Cancer, 68 (S2). https://doi.org/10.1002/pbc.28349
Sagnic, S. (2021). Obesity and Endometrial Cancer. In V. Rao, & L. Rao (Eds.), Role of obesity in human health and disease . IntechOpen. https://doi.org/10.5772/intechopen.99827
Smrz, S. A., Calo, C., Fisher, J. L., & Salani, R. (2021). An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic. American Journal of Obstetrics & Gynecology, 224 (5). https://doi.org/10.1016/j.ajog.2020.10.042
Surveillance Epidemiology and End Results Program. (2021). Cancer stat factors: Uterine cancer . https://seer.cancer.gov/statfacts/html/corp.html
World Health Organization. (2022). Preventing cancer. https://www.who.int/activities/preventing-cancer
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (2018). Cancer nursing: Principles and practice (8 th ed.). Burlington, MA: Jones & Bartlett Learning.
Source: https://www.nursingce.com/ceu-courses/oncology-nursing-ce-course-part-1
0 Response to "Free Continuing Education for Oncology Nurses"
Post a Comment